FDA hopes to apply new sunscreen rules
Ah, the first official day of summer. For procrastinators like me, it’s a signal that I need to start vacation planning in earnest. For an even bigger procrastinator, the FDA, the start of summer was the trigger for announcing (after a couple decades of dithering) its proposed new rules for sunscreens.
Sunscreen makers have long touted their products’ SPF, or sun protection factor. Sun worshippers look for the lowest SPF, heliophobes for the highest. What few people know is that SPF refers only to the amount of protection the product provides against the sun’s ultraviolet B (UVB) rays, and says nothing about protection against ultraviolet A (UVA) rays. UVB rays are the ones mostly responsible for sunburns; UVA and UVB together contribute to premature aging of the skin and skin cancer.
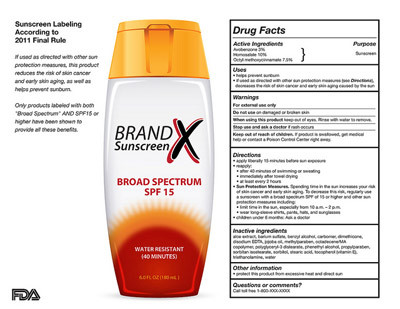
If the FDA’s new rules are approved, beginning next summer a sunscreen’s label must say how much protection it offers against both rays. Products that protect against both UVA and UVB will be labeled “Broad Spectrum” and “SPF 15” (or higher) on the front (see sample label below). The new labels will also say that such products, if used as directed, protect against sunburn and can reduce the risk of skin cancer and early skin aging. Sunscreens that don’t qualify as “Broad Spectrum” or that have an SPF value between 2 and 14 must carry a warning that reads: “Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.”
Some sunscreens already claim to be “broad spectrum,” but these claims haven’t been closely regulated by the FDA. That would change under the new rules.
Other important changes: the highest SPF claim the FDA will allow is “50+” because there’s no evidence of extra protection beyond 50; and sunscreens can no longer claim to be waterproof or sweatproof, or to being a “sunblock.”
The FDA has posted videos covering why some sunscreens work better than others and offering advice on sun safety from FDA dermatologist Jill Lindstrom, M.D.
Applying the rules
Some exposure to sunlight is healthy. It helps set the body’s internal clock, makes vitamin D, improves mood, and more. Too much sun, though, can harm the skin, causing wrinkles and increasing the chances of developing the most common types of skin cancer—basal cell and squamous cell skin cancers. The connection between sun exposure and melanoma, a more deadly type of skin cancer, isn’t quite so straightforward.
Protecting yourself from overexposure to the sun by dressing properly and using sunscreen is one way to keep your skin healthy. The proposed new rules for sunscreen will help you do this in two ways. The FDA will require sunscreen makers to more thoroughly test their products, and the labels will indicate more clearly what the sunscreens can do for you.
What you can do for yourself is apply sunscreen liberally, and often.
About the Author

Patrick J. Skerrett, Former Executive Editor, Harvard Health Publishing
Disclaimer:
As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles.
No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.