
Daily cup of coffee may prevent afib recurrence

Gene-editing therapy lowers harmful blood fats in early study

What is EMDR therapy, and who can it help?

GLP-1 drugs versus bariatric surgery for treating obesity

Trying to lose weight? Be careful not to lose muscle

Two dumbbells, three exercises, and 10 minutes

Easing the emotional burden of IBS

Modify your push-ups to meet your fitness level
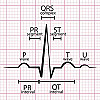
What is long QT syndrome?

Stroke survivors may benefit from very low LDL levels
HHP Medication Safety Watch: February 2021

This list contains selected items from the full FDA list of recalls, withdrawals, and alerts for medicines and certain health products. We've provided links to FDA information for each product and its maker. Unless otherwise noted, these actions apply only to the specific brand name of the product listed. Talk to your healthcare provider before stopping or changing any medicines or treatments that they have recommended for you.
Over-the-counter products and medicines
Dietary supplements may contain undeclared medicines
- Adam's Secret Extra Strength 1500 and Adam's Secret Extra Strength 3000 capsules (maker: Adamssecret.co)
Comment: These products are marketed as nutritional supplements to improve male sexual function. The FDA has found that they may contain sildenafil and/or tadalafil, prescription medicines that treat erectile dysfunction (ED). Sildenafil and tadalafil may cause a dangerous fall in blood pressure, especially if taken with nitrate drugs; other possible side effects include headache, flushing, or stomach upset.
Prescription medicines
Packaging error may lead to incorrect dosing of blood thinner
- Enoxaparin Sodium Injection (maker: Apotex Corp.)
Comment: Enoxaparin is a medicine that is injected under the skin to prevent blood clots in the legs and for certain heart problems. Some batches of this medicine have been recalled because they were packaged with syringes with incorrect markings. This could lead to taking a dosage that is too high (which could cause bleeding) or too low (which could lead to blood clots, heart attack, or other complications).
Medical devices
Oxygen measurement devices may produce incorrect readings
Comment: The FDA has issued a safety communication to highlight possible inaccuracies of pulse oximeters, especially for people of color. These devices clip on to the tip of the finger to estimate oxygen levels. Recent research shows that pulse oximeters may overestimate oxygen levels in as many as 11% to 17% of people of color, but in only about 4% to 6% of white people. These inaccuracies could lead to undertreatment, such as less oxygen therapy than needed; less frequent patient evaluation and monitoring; and underestimating the severity of illness.
Pulse oximeters have been recommended for home use to monitor lung function, a trend accelerated by the pandemic. The FDA recommends increased awareness of this potential problem and avoiding overreliance on pulse oximeter readings, especially for people of color.
Read additional issues of HHP Medication Safety Watch
Disclaimer:
As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles.
No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.

Daily cup of coffee may prevent afib recurrence

Gene-editing therapy lowers harmful blood fats in early study

What is EMDR therapy, and who can it help?

GLP-1 drugs versus bariatric surgery for treating obesity

Trying to lose weight? Be careful not to lose muscle

Two dumbbells, three exercises, and 10 minutes

Easing the emotional burden of IBS

Modify your push-ups to meet your fitness level

What is long QT syndrome?

Stroke survivors may benefit from very low LDL levels
Free Healthbeat Signup
Get the latest in health news delivered to your inbox!
Sign Up

