The new PSA report: Understand the controversy
The day before the findings were even released, The New York Times reported on the long-awaited United States Preventive Services Task Force (USPSTF) recommendation on prostate-specific antigen (PSA) testing. The headline announced "U.S. Panel Says No to Prostate Screening for Healthy Men." The public response was immediate, ranging from approval to confusion and even outrage; the CEO of a prominent prostate cancer advocacy group proclaimed that the USPSTF position "condemns tens of thousands of men to die this year and every year going forward."
Now that the report is available to all of us and we've had time to study it and reflect on its message, we should step back and evaluate PSA screening on its merits. It's a complex issue that requires careful consideration; at the end of the process, even men who disagree about the PSA are likely to agree that The New York Times's front page headline was an unfortunate oversimplification, and that we should all use precise, dispassionate language in discussing this contentious issue. Among other things, we'll see that the USPSTF position is evolutionary, not revolutionary, and that it is a recommendation, not an edict. The Task Force cannot take away a man's right to have a PSA test, nor would it want to. Still, the report is important, and it does point out that before a man decides whether or not to have a PSA test, he should fully understand that screening for early prostate cancer may do more harm than good.
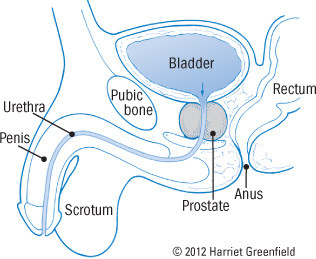
The prostate
|
What is PSA?
At the center of the dispute is a simple glycoprotein (sugar-containing protein) produced by the epithelial cells of every prostate gland, benign or malignant. The prostate secretes PSA in the ejaculate, where its job is to liquefy semen, allowing sperm to swim toward their target. Although PSA is intended for the semen, some of it spills over into the blood, where it can be measured with a simple, inexpensive blood test. But even though the test is simple, interpreting its results is not. And while the test is inexpensive at about $50, it can lead to high downstream costs, both in dollars and health.
One test, several rolesDoctors use blood PSA levels for several different purposes. The test is a very important way to diagnose prostate cancer in men who have symptoms or laboratory abnormalities that raise suspicion of the disease. PSA levels are also used to evaluate the results of prostate cancer treatment. Some doctors even use PSA readings to estimate the severity of benign prostatic hyperplasia, a nonmalignant enlargement of the gland. There is no controversy about these uses of the PSA test — but there is controversy galore about the question addressed in the USPSTF recommendation statement, the use of PSA testing to screen for prostate cancer in men who are free of signs and symptoms of the disease. |
What is the USPSTF?
The Task Force was established by the U.S. Public Health Service in 1984, during the Reagan administration. It is an independent panel of men and women from the private sector who are experts in prevention and evidence-based medicine. Most members are primary care providers in fields such as internal medicine, family medicine, pediatrics, obstetrics-gynecology, and mental health. The panel reviews the scientific evidence behind a broad range of preventive services. It presents its findings in the form of "Recommendation Statements," grading each preventive service as A (strongly recommended), B (recommended), C (no recommendation for or against), D (recommended against), or I (insufficient evidence to support a recommendation).
The recommendations are intended to help primary care practitioners decide which tests and services are best for their patients. The Task Force is widely respected by clinicians, but its recommendations do not dictate either clinical practice or insurance coverage.
PSA permutationsEven before the USPSTF recommendation was unveiled, researchers were aware of the limitations of PSA screening. Several modifications have been proposed, but none has proved superior to PSA itself. PSA travels in the blood in two forms: bound to other proteins or free and unbound. One approach relies on measurements of both the total PSA and the free PSA. Cancer is more likely when the free PSA constitutes less than 25% of the total PSA; the lower the percentage of free PSA, the more likely the diagnosis of cancer. Another refinement depends on serial measurements of PSA, typically at yearly intervals. The PSA velocity reflects the rate of change; researchers suggest that a rise of more than 0.75 ng/ml over the course of a year increases the likelihood of cancer. A similar modification, the PSA doubling time, helps doctors establish the prognosis for patients with prostate cancer; the shorter the doubling time, the worse the outlook. |
In the beginning
Few could have anticipated the PSA controversy in 1966, when the protein was first identified in semen. It rapidly became a favored tool for law enforcement agencies, which used it as a marker for the presence of semen in cases of suspected sexual assault. The next important landmark in the history of PSA came in 1979, when doctors identified PSA in blood. Blood PSA levels were first used to screen for prostate cancer in 1987, and FDA approval for PSA as a screening test followed seven years later.
PSA screening for prostate cancer caught on rapidly in the United States. By now, most men over age 50 have been tested and many have been tested repeatedly. That's no surprise, since Americans have been encouraged to value the early diagnosis of cancer along with the prompt and often aggressive treatments that frequently follow. But while the appeal of PSA screening is understandable, controversy began almost as soon as the PSA test became popular, as some experts worried that testing might do more harm than good.
Although the arguments for and against PSA screening date back to the 1990s, the basic logic behind each position remains largely unchanged. So let's first examine that logic and then review the evidence that has accumulated.
Prostate-specific?For all the uncertainties about PSA, at least we can be sure the name is accurate. Wrong. The protein that bears the name "prostate" has also been detected in other organs, including the liver, pancreas, salivary gland, and breast (even in females). Only tiny amounts of PSA are present in these tissues, far too little to affect a blood PSA test. Still, purists might prefer the name "prostate almost-specific antigen," while wags might suggest "perplexing semantic anomaly." |
The case for PSA screening
The argument for PSA testing is simplicity itself. With an estimated 240,890 newly diagnosed cases in 2011, prostate cancer is the leading internal malignancy in American men — and since the disease took about 33,720 lives in 2011, it is the second leading cause of cancer death in American men.
Early detection of prostate cancer offers the best chance of cure. The PSA blood test is the best currently available way to detect prostate cancer, and the test itself is easy, safe, and inexpensive.
That makes PSA screening sound like a no-brainer. But it's not the whole story.
The case against PSA screening
Even the most outspoken critics of routine PSA screening of healthy men agree that testing does detect a large number of early cancers. But from the very beginning, the skeptics have had three reasons for worrying that early diagnosis may not actually be a good thing.
First, the natural history of prostate cancer itself. The disease has an extremely variable course. In some cases it is aggressive and lethal, but much more often, it's slow-growing and indolent. In round numbers, an American man's risk of developing prostate cancer at some time in his life is at least 30%, yet his risk of developing clinically important disease is about 17%, and his risk of dying from the disease is only 3%. In other words, many prostate cancers are harmless even if untreated; a man is much more likely to die with prostate cancer than from it.
Second, the PSA test cannot tell which cancers are likely to be indolent and which are aggressive. That means routine screening will detect many tumors that would never cause harm, a problem called overdiagnosis. Even worse, since a diagnosis of prostate cancer in the United States usually leads to treatment, PSA testing leads to overtreatment. Despite advances in therapy, significant side effects are common, with erectile dysfunction heading the list and urinary incontinence in second place. So while PSA testing surely saves some lives, it also produces substantial distress in other men who never needed any treatment.
The third issue relates to the test itself. Most American doctors use 4.0 nanograms per milliliter (ng/ml) as a cutoff, accepting results below that as normal and higher values as abnormal. In fact, though, there is no true normal. For example, one important study reported that 17% of men with PSAs between 1.1 and 2.0 ng/ml had prostate cancer, and 23.9% of men with readings between 2.1 and 3.0 ng/ml had the disease. At the other end of the spectrum, about three of every four men with PSAs above 4.0 ng/ml do not have cancer. That's because benign prostatic hyperplasia (BPH), infections, inflammation, and other conditions can boost PSA levels, while many other conditions can lower PSA readings (see "PSA variability").
PSA variabilityFactors that typically produce a substantial and/or sustained rise in PSA
Factors that sometimes produce a small and/or temporary rise in PSA
Factors that typically produce a substantial and/or sustained fall in PSA
Factors that sometimes produce a small and/or temporary decrease in PSA
|
The USPSTF weighs in
With compelling arguments and passionate advocates on both sides, the USPSTF has spent years evaluating the pros and cons of PSA screening for prostate cancer. In its 2002 report, the Task Force gave the test a grade of I; it concluded that there was insufficient evidence to recommend for or against screening.
Needless to say, that did little to guide physicians and their patients, and it did nothing to stem the tide of PSA testing. But as new studies appeared, the Task Force revisited the question in 2008 and again in 2011.
Disclaimer:
As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles.
No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.