Understanding ductal carcinoma in situ
Most women diagnosed with this noninvasive breast cancer are alive 10 years later, and better treatments are emerging.
For the 62,000 women who will be diagnosed with ductal carcinoma in situ (DCIS) this year, the good news is far more important than the bad. While cancer is never a picnic, DCIS is the earliest detectable form of the disease. Some news that sounds "bad" — for instance, that the incidence of DCIS is increasing faster than that of any other type of breast cancer — is encouraging news. It means that more breast cancers are being detected early, while they can be nipped in the bud. Today, with standard treatment, 10-year survival rates for DCIS are approaching 100%, and the treatment is usually not too difficult to tolerate.
What is DCIS?
The name says a lot about the disease. "Ductal" refers to the site of origin, the tiny ducts that form a network connecting the milk-producing structures called lobules. "Carcinoma" indicates a tumor arising in the epithelium, or lining, of the ducts. "In situ" delivers the good news that the tumor is confined to its place of origin; it hasn't invaded the surrounding tissue or metastasized to other body tissues.
The diagnosis of DCIS describes a cluster of cells captured in the process of evolving from normal tissue to breast cancer. The journey is thought to begin with a series of genetic changes in breast cells. At first, these changes stimulate cell growth, resulting in ductal hyperplasia (an overabundance of normal cells), which may begin to fill the duct. Then the cells become distorted and look abnormal under a microscope. At this second stage of change, called atypical ductal hyperplasia, the cells' capacity for growth is further increased. DCIS proper is a third step in the process, in which a cluster of abnormal cells has filled the duct but hasn't broken through its walls. If it does breach the walls, it's called invasive breast cancer.
Carcinogenesis, the process by which cancer arises, may not take place precisely in these orderly steps. However, pathologists have developed these classifications as indicators of the progression of the disease.
|
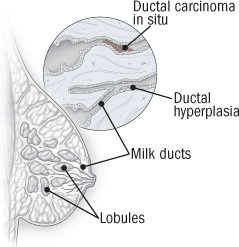
Anatomy of ductal carcinoma in situ
Ductal carcinoma in situ (DCIS) is an overgrowth of abnormal cells in the milk ducts of the breast. It starts with the proliferation of normal cells lining the milk ducts (ductal hyperplasia); next, the cells within the duct become abnormal and rapidly multiply (atypical ductal hyperplasia); finally, abnormal cells fill the duct (DCIS). Invasive ductal breast cancer occurs when abnormal cells break out of the milk duct. |
Diagnosing DCIS
Like other types of cancer, DCIS is usually diagnosed by a team of medical professionals (including radiologists, surgeons, and pathologists), using the following techniques:
Mammography. In a sense, increased use of mammography is responsible for the increase in DCIS, because it has increased detection. Confined to the ducts, DCIS tumors are often too small to cause symptoms or to be felt on a breast exam. Before mammograms became routine in the late 1970s, DCIS was usually discovered incidentally during a biopsy or autopsy, and it was thought to be rare, constituting fewer than 1% of breast cancers. Today, DCIS is likely to be identified during an annual mammogram that reveals tiny calcium deposits — microcalcifications — which appear as lines or clusters on an x-ray image and are sometimes associated with cancer. As mammography improves, so does the diagnosis of DCIS. In 2005, the last year for which statistics are available, DCIS accounted for more than 20% of newly diagnosed breast cancers.
Magnetic resonance imaging (MRI) is now increasingly used in breast imaging, but it hasn't yet been found significantly better than mammography in screening for DCIS.
Biopsy. Once DCIS is suspected, a biopsy is needed to determine whether cancer is actually present and, if so, the extent of the disease. These days, biopsies are more likely to be performed in the radiology suite than in the operating room. In the most commonly used procedure, stereotactic core biopsy, a large needle or thin vacuum tube is guided by ultrasound into the region of the breast containing microcalcifications to take a tissue sample. However, when mammography has indicated large areas of microcalcification, a surgical biopsy may be recommended.
Pathology. Pathologists examine the biopsy sample to determine how far the tissue has strayed from normal breast tissue. They look at the structure and arrangement of the cells under a microscope and may test the sample to determine the presence of receptors for estrogen and progesterone or abnormalities in genes associated with cancer.
By considering the features and growth pattern of the cells, they will characterize the disease as low grade, intermediate grade, or high grade — a classification that reflects how different the tumor cells look from normal cells and how quickly the tumor is likely to grow.
Treating DCIS
The data are limited on treating modern DCIS — which is identified by screening mammogram instead of being found rarely in large tumor masses. Until the 1980s, DCIS was routinely treated in the same way as most invasive cancers — with mastectomy.
That situation began to change after a large ongoing study, the National Surgical Adjuvant Breast and Bowel Project (NSABP), reported in 1983 that women with small invasive tumors who underwent lumpectomy followed by radiation were just as likely to survive as the women who underwent mastectomy. Physicians naturally assumed that the same approach could also work for patients with DCIS, and that assumption has been confirmed by large studies from the NSABP and the European Organization for Research and Treatment of Cancer.
Treatment decisions
Today, the results of mammography and biopsy determine the choice between mastectomy and lumpectomy. DCIS is never an emergency, so you can take a few weeks to weigh your options, which include the following:
-
Breast-conserving surgery (lumpectomy) is often recommended when DCIS is limited to one site and the tumor can be removed with a clear margin — several millimeters — of healthy tissue.
-
Radiation therapy is recommended for all women who have had breast-conserving surgery, because it reduces the chance of recurrence after surgery from 30% to 15%. The standard procedure is full-breast radiation administered in a hospital or center five days a week for five to eight weeks. Newer approaches are on the way (see "Future directions in DCIS").
-
Tamoxifen (Nolvadex) may further reduce the recurrence rate. In a randomized controlled NSABP study reported in 1999, women who received tamoxifen after surgery and radiation for DCIS were only half as likely to have a recurrence within five years, compared with similarly treated women who got a placebo.
-
Mastectomy is associated with a 10-year disease-free survival rate of 98%. It's usually reserved for women who have DCIS in more than one part of the breast or in cases where removing the tumor and a margin of healthy tissue around it would require a disfiguringly large incision. Mastectomy is also recommended for women who have a recurrence of DCIS or invasive cancer at the same site. Some women may choose mastectomy because they want to avoid undergoing radiation, or because they want to reduce their risk of recurrence to the lowest level possible.
-
Because the risk of metastasis is so low, lymph node biopsy is not required for diagnosing DCIS, and adjuvant chemotherapy is not necessary in treating it.
|
Lobular carcinoma in situ, the other preinvasive disease Despite its name, lobular carcinoma in situ (LCIS) technically isn't breast cancer, and it may not even be a precancerous condition. However, it is generally considered a risk factor for the disease. Although a handful of small studies (most including fewer than 200 women) have suggested that LCIS doesn't directly progress to invasive cancer, women with LCIS are four times more likely than average to develop invasive ductal cancer and 18 times more likely to develop invasive lobular cancer. LCIS is a proliferation of abnormal cells in the milk-producing structures (lobules) of the breast. The incidence of LCIS has been rising steadily since the late 1980s, mostly among women ages 50 and over. And since 2000, incidence has increased only among those ages 50 to 69 — the group most likely to have regular mammograms. LCIS doesn't produce lumps that can be felt during breast exams or microcalcifications that appear on a radiology screen. It's usually discovered by chance during a biopsy for a benign breast condition or an invasive form of cancer. It occurs in an estimated 0.5% to 3.8% of benign breast biopsies, but no one knows for certain how common it really is. LCIS usually arises in several lobules of both breasts, so lumpectomy isn't an option. Instead, LCIS is managed in these ways:
|
Future directions in DCIS
DCIS research is directed mainly at improving treatment and, above all, at preventing progression to invasive disease. As researchers continue to study the pathology of DCIS, they are finding that certain tumor characteristics help predict the treatment most likely to reduce the chance of recurrence. For example, some forms of breast cancer require estrogen in order to grow; tumors that do are termed estrogen receptor–positive (ER-positive). Tamoxifen belongs to a class of drugs called selective estrogen-receptor modulators (SERMs), which act by blocking estrogen receptors. Tamoxifen is more likely to prevent a recurrence in women with ER-positive DCIS than in women with ER-negative disease.
The use of aromatase inhibitors, which block estrogen production in the peripheral tissues and breast tissue, is being investigated in a trial of postmenopausal women with ER-positive DCIS. For women whose DCIS is ER-negative but who have the HER-2/neu gene, researchers are exploring the use of trastuzumab (Herceptin) and lapatinib (Tykerb), which block the tumor growth factors produced by that gene.
Another promising area of investigation involves the short-term use of chemotherapy between diagnosis and surgery to alter the DCIS, so that tissue from the surgical resection can be used by researchers to assess molecular as well as pathologic evidence of response. Agents that can induce responses in the "right direction" — for example, slow or stop the growth of abnormal cells — may then be further evaluated for their potential in treatment or prevention.
A new way to administer radiation that is showing some promise in clinical trials is accelerated partial breast irradiation, in which the tumor site alone is treated for five days with a lighter dose of radiation. In another approach, intraoperative radiation therapy, a one-time dose of radiation is delivered to the involved area of the breast after the tumor has been removed but before the incision is closed.
The good news about DCIS
DCIS is sometimes classified as Stage 0 of breast cancer, the earliest stage of the disease. The question for women with this diagnosis is not "Will I live?" but "How much treatment will I need?" One of the biggest risks today is overtreatment. That, too, is changing, as researchers get better at distinguishing the types of tumors that can be subdued without extensive surgery or radiation. DCIS is one cancer that can truly be considered curable.
If you have DCIS, you might consider entering a clinical trial. You would get the best available care and might benefit from a new type of therapy or approach. At the very least, you would be contributing to much-needed knowledge about this condition. Check the National Cancer Institute's registry of clinical trials at www.cancer.gov/clinicaltrials for a site near you.
Disclaimer:
As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles.
No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.